Is Compounded Semaglutide Legal?
Updated
Mar 15, 2024
Published
Jan 12, 2023
Semaglutide is patented so only one company can make it, right? Well, it's more than that.
You May Have Heard of Semaglutide…
The once-a-week injectable GLP-1 receptor agonist that has taken the internet by storm. It helps to lower blood sugar levels by stimulating the production of insulin and slowing down the absorption of glucose in the gut. It is marketed under the brand name Ozempic® for Type-2 Diabetes, and as Wegovy® for weight loss by the Novo Nordisk® company.
Touted as the solution for obesity by everyone from doctors to celebrity CEO, Elon Musk. But this fame has come at a cost and rising popularity has led to shortages of Semaglutide across the country, and now many people can’t get access to this important drug that has countless benefits, including better glucose control and substantial weight loss. Given this issue, many people have been turning to compounded semaglutide and many are wondering if this compounded semaglutide is legal?

Is Semaglutide Patented?
It is 100% true that Ozempic® and Wegovy® are patented drugs. Specifically, Novo Nordisk® owns the patent on the active pharmaceutical ingredient (API) in both Ozempic® and Wegovy®, which is Semaglutide. So, that must mean nobody else can make Semaglutide until the patent expires… right? Not exactly.
What is a Patented Drug?
A patented drug is a medication that is protected by a patent, which is a legal monopoly granted to the inventor or assignee of the drug. This means that only the patent holder is allowed to produce, sell, and distribute the drug for a certain period of time.In the United States, the length of time that a drug patent lasts depends on the type of drug and when it was approved by the US Food and Drug Administration (FDA). Generally, drug patents last for 20 years from the date of filing. This isn’t always how long a patent lasts though.
For example, if a drug is approved under the FDA’s Orphan Drug Act, the patent term may be extended by up to five years. This is because the Orphan Drug Act provides incentives for pharmaceutical companies to develop drugs for rare diseases, which are diseases that affect fewer than 200,000 people in the US. Additionally, if a drug is subject to FDA regulatory review, the patent term may be extended by up to five years to compensate for the time the drug company spent in the regulatory review process.
It’s also worth noting that, in some cases, a drug company may be able to extend the patent term of a drug by obtaining additional patents for new uses, formulations, or methods of administration of the drug.
However, there are certain circumstances in which a patented drug may be compounded by third-party compounding pharmacies.
Is Compounded Semaglutide Legal? Yes!
One of the main reasons that a patented drug may be compounded is to address a shortage of the medication. A drug shortage can occur for a variety of reasons, such as production problems, manufacturing delays, or increased demand for the drug. In the case of Semaglutide, the drug shortage has been so severe that patients are unable to access the medication they need to manage their condition.
When a drug is on the FDA Shortage List, the FDA may allow compounding pharmacies to produce a compounded version of the drug to help alleviate the shortage.The FDA has a list of drugs that are in shortage, which is updated regularly.
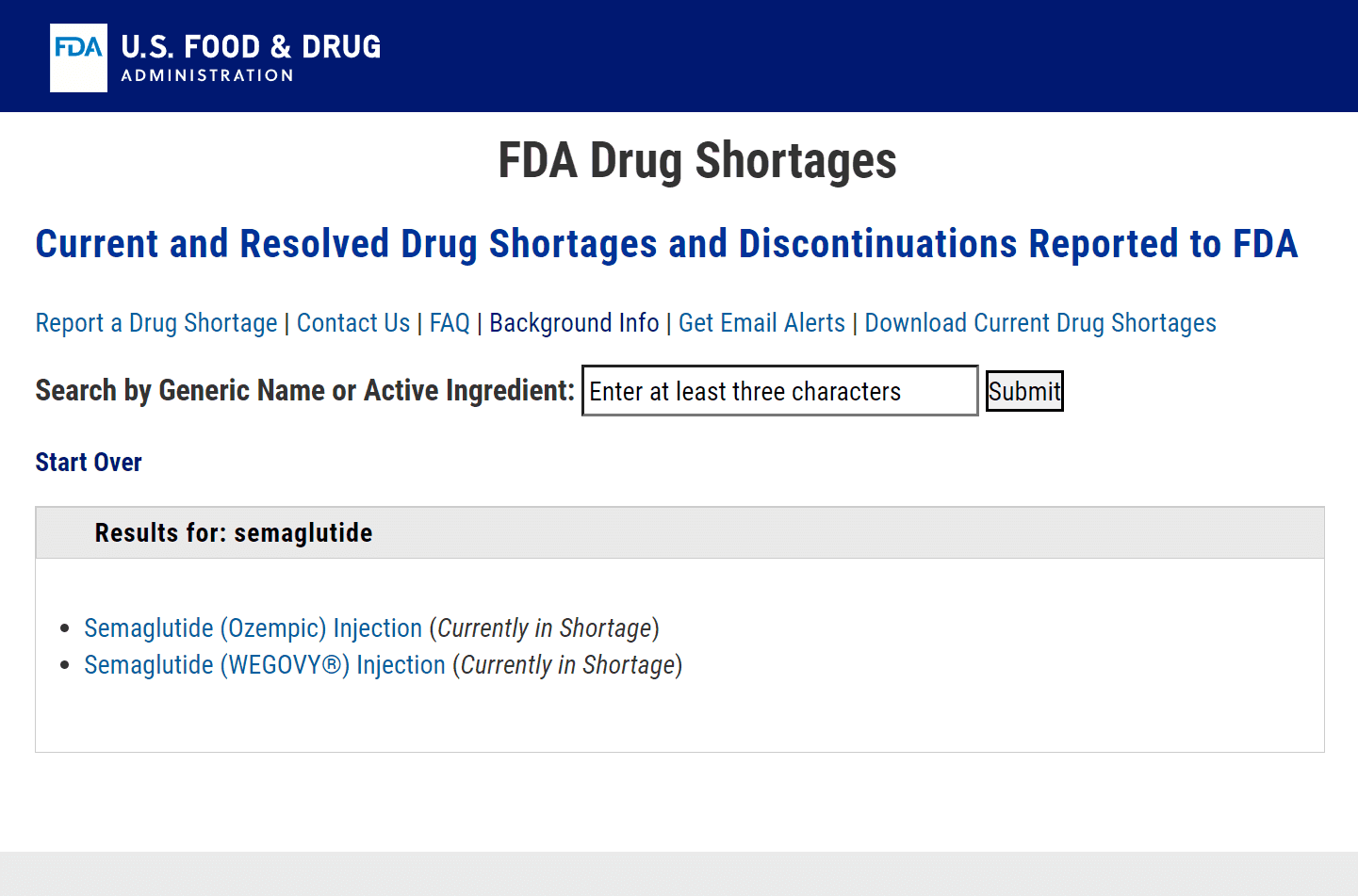
The FDA must first determine that there is a shortage of the drug and that there are no suitable alternatives available. Additionally, the FDA must also ensure that the compounded version of the drug is safe and effective, and that it is being produced under appropriate conditions. This can be met by ensuring the drug is produced exclusively in an FDA-licensed compounding pharmacy.
Other Reasons for Compounding Patented Drugs
Another reason that a patented drug may be compounded is to provide a customized medication for a specific patient. For example, a patient may have an allergy to an ingredient in a drug, or they may require a different dosage than what is available on the market. In such cases, a compounding pharmacy may be able to create a customized version of the drug that is tailored to the patient’s needs.
Additionally, if a specific drug is not yet available in a specific form, such as a liquid form for pediatric patients or a long-acting form for some chronic conditions, compounding pharmacies may be able to create these formulations. This can be done by compounding a drug that is already FDA-approved but not available in the desired form.

The Future of Compounded Semaglutide
There is currently no known end-date for the Semaglutide shortage. When the shortage does end, compounding pharmacies will be prohibited from producing Semaglutide as it relates to the drug shortage. However, compounding pharmacies may still be allowed to create customized dosage forms or alternative delivery methods of Semaglutide based on the specific needs of individual patients.
Compounding pharmacies play a vital role in the United States healthcare system, and their services should be valued and supported.
Do you need Semaglutide?
If you need compounded Semaglutide, Henry Meds may be able to help. We have created a platform that connects patients, providers, and compounding pharmacists in one place so that everyone can afford the lifesaving medications they need.
Read more from our blogs
The Side Effects of Rybelsus
Apr 23, 2024
Everything you need to know about oral Semaglutide and the possible side effects.
Semaglutide's Role in Non-Diabetic Weight Management
Apr 19, 2024
Learn more about semaglutide for weight loss in non-diabetics.
Compounding Pharmacy Semaglutide Cost: What to Expect
Apr 16, 2024
Compounding pharmacy semaglutide cost and what you can expect.
Back to blog




